Research lines
Our overarching goal is to advance or understanding of the human heart and offer novel therapeutic approaches to treat congenital heart disease and cardiovascular disorders.
|
|
Synthetic heart development and congenital heart defects. During development, an exquisitely orchestrated series of biological processes lay down the map for the entirety of our bodies, and carry it out to perfection. However occasionally errors occur (due to mutations, environmental factors, and other causes) and lead to congenital defects. Congenital heart defects (CHDs) are the most common birth defect in humans and affect 1% of all newborns. Certain conditions such as obesity, diabetes, infections or drug use can increase this risk much further.
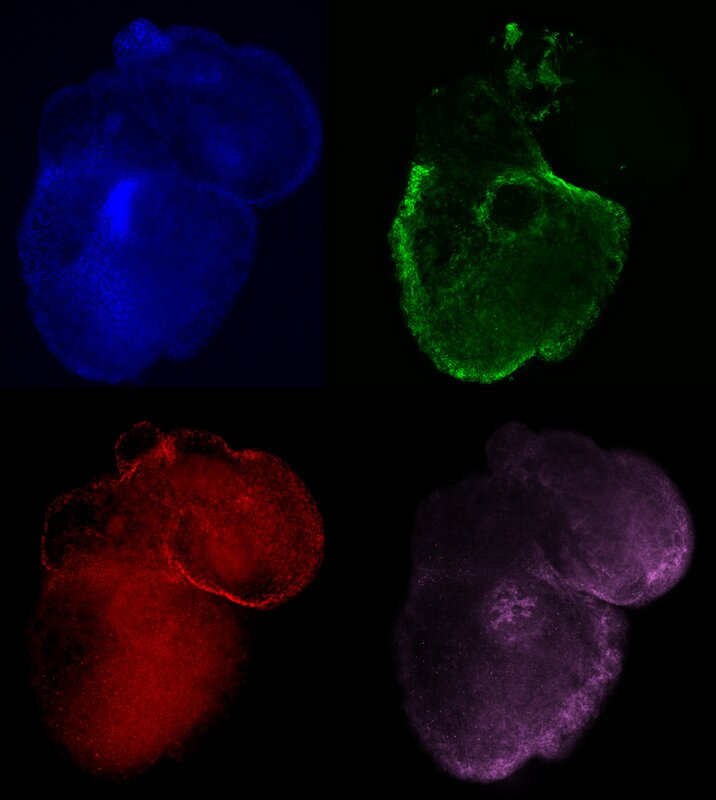
To tackle CHDs, we are reverse engineering human heart development on a dish with the use of pluripotent stem cells, creating heart organoids or mini-hearts. By recapitulating these aspects of heart development in vitro, under fully controlled conditions, we can dissect gene networks and morphological changes that give rise to specific parts of the heart to understand and prevent CHD, such as single ventricle defects. Furthermore, we can also use these mini-hearts as models to study the exposure to environmental conditions and other factors that are very poorly known. Another upside of reverse engineering heart development is the ability to grow human hearts in vitro from pluripotent stem cells, without the need for scaffolds or animal tissues. Although this is still far out of reach, we have made seminal advances in growing human embryonic and fetal-like hearts on a dish, and we expect to continue improving on these technologies in the near future. One day these synthetic hearts might be mature enough to use as fully compatible transplants (since they can be grown from the patient's own cells). |
|
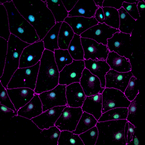
Cardiac stem cells, inflammation and heart regeneration. Unlike humans, many vertebrates exhibit a remarkable capacity to regenerate their heart, including some mammals (neonatal mice), amphibians (newt) and fish (zebrafish). In cases where regeneration occurs, it happens either by mobilizing endogenous stem cell pools, such as epicardial cells, or by dedifferentiation of tissue resident cells, the best characterized model in mammals being cardiomyocytes in the heart. Regeneration in this organism occurs with no detectable downside effects and complete functional restoration. The molecular events behind these processes are complex, and involve a multitude of cells types and signals, including inflammation, epigenetic remodeling and reactivation of developmental pathways.
In our lab, we want to establish human-based models to study and model heart regeneration. Since cardiovascular disease constitutes the first cause of death in the developed world, ahead of cancer, this is a topic of enormous health impact in our society. One of our topics of interest is how inflammatory signaling, cell dedifferentiation and progenitor cell mobilization occur. For studying regeneration we use human pluripotent stem cells differentiated in human heart tissues of interest and zebrafish. The study of regeneration can offer valuable clues to develop reprogramming therapies to treat cardiovascular injury. |
|
|
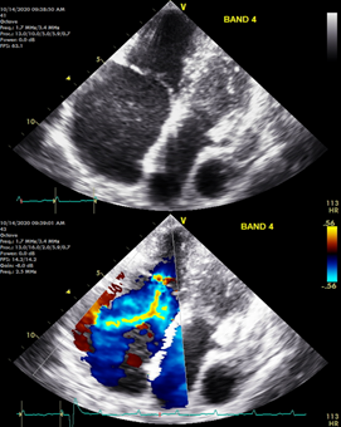
Cardiac tissue engineering. The heart is a highly robust and complex mechanical pump that uninterruptedly pushes blood around the body for many decades of life. There is critical need for replacement parts for damaged hearts due to the prevalence of cardiovascular disease. Using a combination of tissue engineering and stem cell biology approaches we are growing human heart parts in vitro that might one day be useful for transplantation. This in vitro tissue and organ-like systems can also be used as models to study cardiovascular disease in a dish to develop new therapies.
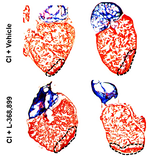
In one of our current projects, we hare growing human heart valves to understand and treat valvopathies, such as tricuspid regurgitation. To carry out its function efficiently, the heart has chambers separated by soft tissue structures akin to floodgates (the heart valves) that control the entry and exit of blood from each chamber. Heart valves undergo continued mechanical wear and tear as they work in our bodies. This damage that is constantly repaired by cells living in those valves. In some instances, however, valve cells become dysfunctional, either by producing too much tissue or by proliferating in excess. This process is called pathologic remodeling and leads to valve malfunction, a condition that affects the mechanical ability of the heart to pump blood efficiently and leads to multiple life-threatening health problems. Tricuspid valve malfunction, clinically referred to as functional tricuspid regurgitation, is a public health problem in the US but cannot be treated efficiently due to a lack of understanding of the underlaying causes. In our lab, we want to study how tricuspid valve regurgitation develops by identifying the alterations present in valve cells, with the goal of using this knowledge to make new drugs for treating patients. We create the valves from human pluripotent stem cells and engineered them in a dish. The valves created are not only useful as models, they might also be suitable for transplantation one day. |
Proudly powered by Weebly